The pharmaceutical industry is constantly evolving, driven by the need to develop new and effective drugs, treatments and therapies for a growing range of diseases that address unmet medical needs and improve human health. Many pharma companies are undertaking digital transformation initiatives across their operations, including the introduction of intelligent lab systems. These systems have the potential to modify access to data and the integration of disparate informatics platforms that secure a return on investment (ROI) and enable effective change management strategies in the lab.
This rapid pace of innovation has placed significant demands on R&D lab engineers, who play a vital role in developing and optimizing laboratory processes. However, the adoption of new technologies and methodologies can often be met with resistance from lab engineers, leading to delays and inefficiencies in the R&D process.
In this blog post, we will explore some of the main reasons why pharma labs are facing acceptance challenges from lab engineers, and what can be done to overcome them.
As data collection and analysis within pharma redefine the function of the lab, scientists are experiencing challenges as they attempt to turn against a deluge of data and utilize intelligent lab systems to alter access to data. Also, we need to first understand the challenges faced by the lab engineers. They face many challenges in adopting new technologies and processes that could enhance their productivity and efficiency.
Understanding resistance factors
In pharmaceutical R&D, lab engineers conduct experiments, analyze data and develop new drugs. However, they are often overworked and understaffed, with tight deadlines and high expectations. This can lead to stress, burnout and a reluctance to accept new technologies that could add to their workload.
Several factors contribute to the resistance of R&D lab engineers to adopt new technologies:
- Time pressure: R&D in the pharmaceutical industry is typically driven by tight deadlines and high expectations. Lab engineers often have immense pressure to deliver results within limited timeframes, which can lead to rushed experiments, shortcuts and potential errors. This can have a detrimental impact on the quality and reliability of research data, ultimately delaying the development of new drugs and therapies.
- Fear of change: Lab engineers are often accustomed to working in a traditional environment with well-established procedures. Introducing new technologies can disrupt their established workflows and comfort zones, leading to apprehension and reluctance to accept change.
Additional workload: New technologies can be viewed as more work. Lab engineers often hesitate to adopt new technologies because they perceive them as adding more work to their already demanding workloads. They may worry about the time and effort required to learn new systems, the potential for disruptions to their existing workflows and the possibility of new errors or mistakes. This can make it difficult to convince lab engineers of the benefits of new technologies, even if those technologies promise to improve efficiency or productivity.
Examples of how new technologies can be perceived as additional work:
- A new Laboratory Information Management System (LIMS) may require lab engineers to learn a new interface and adapt to new workflows, which can be time-consuming and disruptive.
- A new data analysis tool may require lab engineers to learn new statistical methods or software, which can be overwhelming.
- A new automation system may require lab engineers to troubleshoot and maintain new equipment, which can be a burden on top of their existing responsibilities.
- Non-scientific activities consume valuable time: In the bench, lab technicians often spend a significant portion of their time on non-scientific activities that do not directly contribute to research progress. These activities may include:
- Device connectivity: Integrating and managing too many laboratory instruments and devices can be a significant challenge for lab engineers. The need to establish and maintain consistent connections between these devices, often from different manufacturers with proprietary protocols, can lead to compatibility issues, data loss and disruptions in research workflows. This can be particularly problematic when dealing with complex experiments that involve multiple instruments and data streams.
- Moving data between devices and systems: Pharmaceutical research often involves large amounts of data that must be transferred between various instruments, computers and databases. This process can be time-consuming and error-prone, especially if data is transferred manually or through incompatible formats.
- Logging into multiple applications: Lab engineers often need to access multiple applications to perform their daily tasks, each with its own login credentials and security protocols. This can be a tedious and time-consuming process, especially when dealing with complex passwords or authentication systems.
- Resolving technical issues: Lab instruments and equipment can malfunction or experience technical glitches, often requiring lab engineers to spend time troubleshooting and resolving these issues. This can disrupt research workflows and divert attention away from core scientific activities.
- Lack of user-friendly interfaces: Many laboratory applications, particularly those developed for specialized equipment, are often designed with a focus on technical functionality rather than being user-friendly. Complex interfaces and lack of intuitive design can make it difficult for lab engineers to quickly navigate and access the information they need. This can lead to frustration, delays and potential errors in data entry and analysis.
- LIMS manages and organizes laboratory data. However, traditional LIMS solutions often lack flexibility and adaptability, making it difficult to integrate new technologies or adapt to changing research protocols. This can lead to data silos and hinder the efficient flow of information between different research teams and departments.
- Electronic Laboratory Notebook (ELN) systems are essential for documenting and storing experimental data in a secure and searchable format. However, many ELN systems are awkward to use and lack the ability to capture all aspects of the experimental process, including detailed notes, images and attachments. This can lead to incomplete or inaccurate documentation, which can hinder reliability and impede scientific progress.
- Lack of training and support: Effective adoption of new technologies requires adequate training and support for lab engineers. Unfortunately, many organizations struggle to provide comprehensive training and guidance, leaving engineers feeling unprepared and hesitant to transition to new methodologies.
- Mistrust of vendors: Lab engineers may distrust technology vendors, perceiving them as motivated by profit rather than in the best interests of the laboratory. This mistrust can lead to skepticism about new technologies and a reluctance to trust vendors' claims.
- Concerns about data security and integrity: Pharmaceutical laboratories handle sensitive data, and lab engineers are understandably cautious about adopting technologies that could compromise data security or integrity. They may question the robustness of new systems and the potential for data breaches.
Complexity of experiment processes
The complexity and diversity of the processes and workflows in pharma laboratories are challenging. Unlike other industries, where automation can be applied to standardized and repetitive tasks, pharma laboratories deal with a wide range of experiments, samples and data types. Each process may require different equipment, protocols and quality standards, making it difficult to integrate and optimize automation systems across the entire laboratory. Lab engineers may prefer to rely on manual methods that are more flexible and adaptable, rather than embracing automation systems that may not suit their specific needs or preferences.
Many lab engineers log in and out of many applications to execute an experiment because they often need to use multiple applications to execute an experiment. For example, during a lab visit, a scientist and lab technician may need to log in and out of 10 applications to execute one experiment. Imagine the time lost and the frustration. However, cybersecurity rules implemented by the security and compliance IT team also disturb ongoing tasks. For example, patches are pushed to systems while lab engineers are running experiments, or a Microsoft Windows lock screen appears after a given time of inactivity on the equipment computer running the experiment.
Complexity and cost of automation
Another challenge for pharma laboratories wanting to adopt automation systems is the shift in the role of lab engineers. They are now required to have a deeper understanding of automation systems and be able to troubleshoot issues that arise. There is also a significant upfront cost of obtaining new equipment. Commercially available equipment is expensive, and machinery tailored to specific purposes is even more money. Moreover, automation systems require a high level of technical expertise and maintenance, which adds to operational costs and risks. Lab leaders may be reluctant to invest in automation systems that are not proven to deliver an ROI or that may become obsolete in a rapidly changing environment.
Culture and mindset of innovation
Yet another challenge for pharma laboratories to adopt new technologies and processes is the culture and mindset of innovation. Pharma laboratories are often driven by curiosity and creativity rather than efficiency and productivity. Lab engineers may value the freedom and autonomy to explore new ideas and hypotheses rather than following predefined and standardized procedures. They may also be more comfortable with trial and error rather than relying on data and analytics. This may make them resistant to change or skeptical of new technologies and processes that constrain their innovation potential or introduce new uncertainties.
Furthermore, pharma laboratories are often subject to strict regulations and ethical standards, which may limit the ability or willingness to adopt new technologies and processes. Lab engineers may be concerned about the safety, quality and validity of the results obtained from new technologies and processes, especially if they are not well understood or validated. They may also be wary of the legal and social implications of using new technologies and processes that may affect the privacy, security or ownership of the data and intellectual property generated in the laboratory.
Solutions and strategies for adoption
Despite these challenges, pharma laboratories can benefit from adopting new technologies and processes that improve performance and outcomes. To overcome these challenges from lab engineers, pharma laboratories must develop a clear long-term lab-evolution strategy blueprint and use a holistic and strategic approach.
HCLTech’s lab experts can build a lab blueprint strategy to demonstrate the value of efficiency by adding technology value, which will save time for scientists and lab technicians to focus solely on research tasks.
- Value proposition and business case for new technologies and processes: Pharma laboratories must demonstrate how new technologies and processes can enhance the quality, speed and cost-effectiveness of their research and development activities and create a competitive advantage or differentiation in the market. They also need to quantify the expected benefits and costs, and measure the actual impact and ROI of the new technologies and processes.
- Showcase success stories: Highlight successful case studies where new technologies have improved productivity, reduced costs or enhanced innovation. This can demonstrate the tangible benefits of adoption and encourage other engineers to embrace change.
- Prioritize education and training: Provide comprehensive training and support to lab engineers, ensuring they understand the benefits and applications of new technologies. This training should not only cover the technical aspects but address practical considerations and potential challenges.
- Standardize device connectivity: Standardizing protocols and protocol adapters can simplify device connections and reduce compatibility issues. The use of HCLTech middleware can help fix this issue.
- Empower engineers with ownership: Give lab engineers ownership over the adoption process, allowing them to participate in decision-making and contribute their expertise. This sense of empowerment can foster a more positive attitude toward change and encourage active involvement.
- Design user-friendly interfaces: Prioritizing user-centered design principles can make applications more intuitive and easier to navigate. HCLTech can help pharma R&D labs build a user-friendly layer that streamlines login and logout procedures, saving time and reducing the risk of errors. Using flexible user friendly UIs to access all lab applications will save time and gain efficiency.
- Continuous improvement: Encourage a culture of continuous improvement, where lab engineers are constantly evaluating and adapting their practices. This mindset can make adoption more organic and sustainable as engineers constantly seek new ways to optimize their workflows.
- User experience and engagement: Pharma laboratories need to design and implement the new technologies and processes in a user-friendly, intuitive way that aligns with the lab engineers' needs and preferences. They also need to provide adequate training and support and solicit feedback and suggestions from the lab engineers to ensure their satisfaction and adoption. Moreover, they need to create a culture and mindset of innovation that encourages and rewards the lab engineers for experimenting and learning from the new technologies and processes.
- Integration and optimization of the new technologies and processes: Pharma laboratories must ensure that the new technologies and processes are compatible and interoperable with existing equipment, protocols and data systems. They also need to optimize the new technologies and processes to achieve the best performance and efficiency and to avoid duplication or redundancy. Furthermore, they need to comply with the relevant regulations and ethical standards and ensure the security and integrity of the data and intellectual property generated by the new technologies and processes.
Conclusion
Pharma laboratories face adoption challenges from lab engineers due to the complexity and cost of automation and the culture and mindset of innovation. Addressing the adoption challenges faced by R&D lab engineers is crucial for pharmaceutical organizations to maintain a competitive edge in the rapidly changing landscape of drug discovery.
However, by adopting a holistic and strategic approach, pharma laboratories can overcome these challenges and leverage the new technologies and processes to enhance their productivity and efficiency and, ultimately, to improve human health.
HCLTech has developed a comprehensive, methodical and flexible framework that is designed to ensure laboratory digitization is a success by maximizing business value for your organization
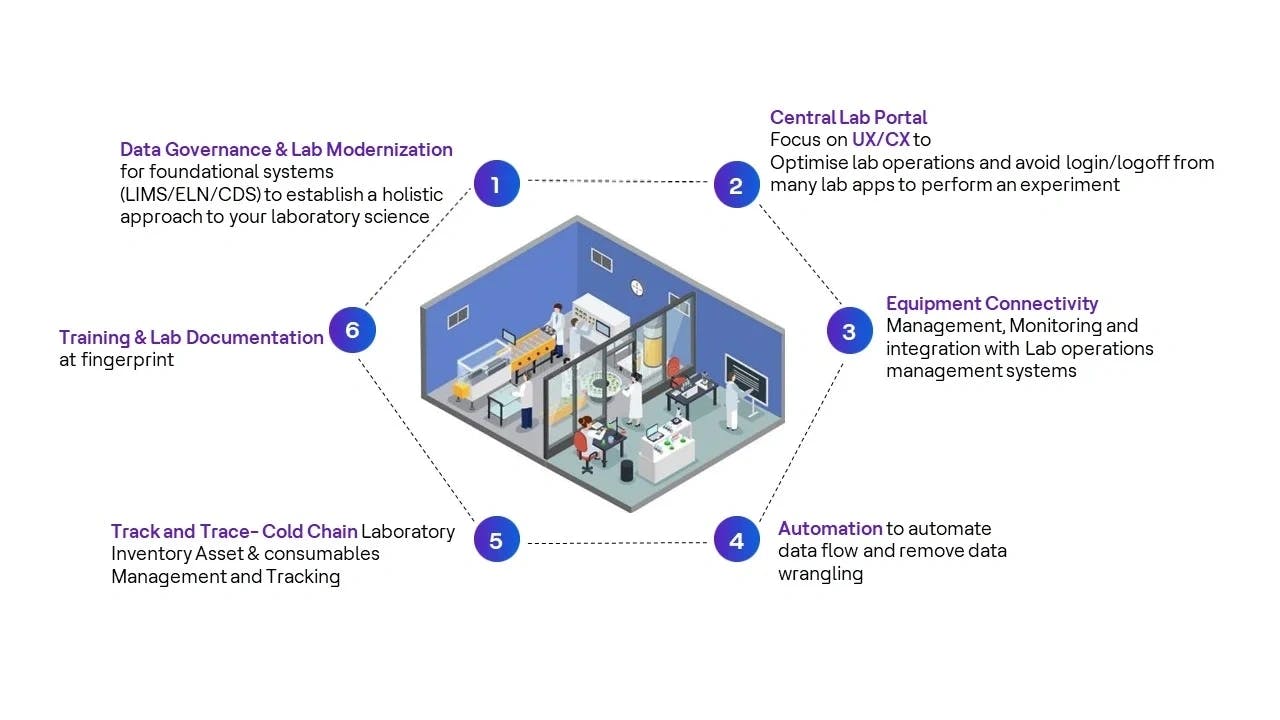
Image 1: HCLTech SmartLabs framework