Industry Context
Pharmacovigilance (PV) is widely used to improve patient care and the safety of medications by detecting and communicating problems promptly. Case processing, a fundamental PV activity, detects safety concerns, provides data for analysis of adverse effects of medications, and facilitates periodic assessment of the benefit-to-risk ratio of medications.
Data processing precision and quality are critical for ensuring correct analysis and prompt corrective actions, which enable safe drug use and safeguard patient health. Adhering to case processing conventions, quality checks (QCs) play a vital role in determining the safety profile of medications by defining the completeness and accuracy of source documents (or information provided by patients).
The challenges of traditional case processing
In traditional case processing, it is essential to weave QCs into multiple workflow processes in the end-to-end PV value chain. In addition to the QC workflow, QCs are used for subsequent workflows: medical assessment, pre-submission, post-submission (retrospective QCs), signal detection, and aggregate reporting. This results in redundant manual work and opens the possibility for inconsistencies in submissions, making it a challenge for PV departments to meet KPIs. PV departments also face challenges with increasing data volume and case complexity. These challenges pose compliance risks if errors are identified, potentially leading to inspections and financial penalties for marketing authorization holders and/or pharma companies.
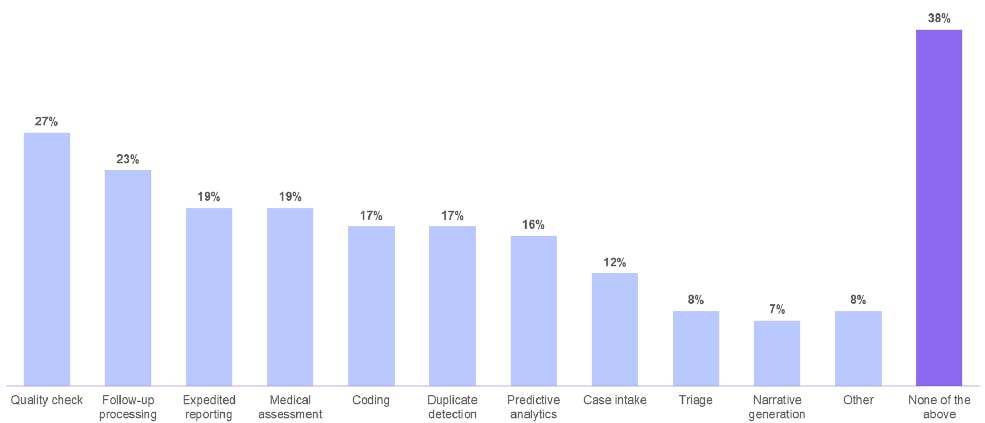
Oracle survey: The data challenges of pharmacovigilance
Possibility of leveraging AI in case processing
Pharma’s seek a solution that improves the quality of information that goes into QCs, reduces case processing costs, and complies with continually changing regulations.
In a survey conducted by Oracle, 62% of respondents said they are actively implementing or planning to implement AI in adverse event case processing. That’s because AI facilitates the workflow steps of quality checks, follow-up processing, expedited reporting, and medical assessment.
As PV departments continue to adopt next-gen technology by leveraging AI, they will deliver high-quality QCs, reduce costs through efficient case processing and enhance compliance.
HCLTech’s Automated Case Quality Check (ACQC) solution
An AI or ML-enabled solution built on an analytical and AI stack, a rule-based engine, NLP, a self-learning module, semantic analysis, and sentimental analysis, Automated Case Quality Check (ACQC) helps PV departments achieve case quality with the ‘first-time-right’ concept.
The highly configurable, plug-in ACQC solution automatically generates a QC before a case can be moved to each subsequent processing step. The solution can be used as a plug-in with safety databases, safety applications, and case processing practices. With the elimination of the traditional workflow, the QC responsibility will rest with the case processor who is routing the case from one workflow to another, as the system automatically forces the generation of a quality report before the case can be moved to the subsequent processing step.
How ACQC works
Case processors trigger ACQC to review case quality at the click of a button. They can also trigger it at case lock, which enables ACQC’s back-end engine to make suggestions to the case processor by checking the client guidance document for the case type.
ACQC produces a calculated quality score (CQS) for each case. If the CQS is greater than the acceptable quality score (AQS), which is based on business requirements and SOPs, the solution updates the case with the CQS. If the CQS is less than the AQS, the case processor can check the suggestion(s) provided by the ACQC solution and accept or reject them. If the case processor rejects a suggestion, he or she gives feedback so that the solution can leverage ML to improve future suggestions.
Depending on the case update, the solution either submits the case to the next step or locks it from being submitted.
The solution provides real-time data visualization for error trend analysis and generates a quality analysis summary report on demand.
Advantages of ACQC
Adopting ACQC helps identify potential case quality issues early, rectify errors, and increase regulatory submissions' quality. This results in a timely qualitative analysis of the potential safety risks of the product or drug.
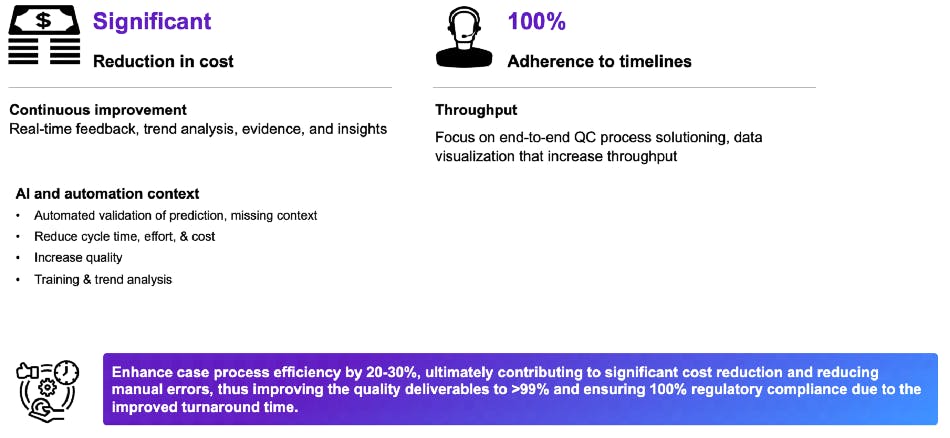
ACQC brings efficiency by eliminating redundant and manual tasks in the PV value chain, which enhances case process efficiency by 20 to 30%. ACQC reduces errors, increasing the quality of deliverables to better than 99%. Automated validation of prediction significantly reduces cycle time, cost, and effort.
The solution ensures 100% adherence to timelines with continuous real-time feedback, data-driven insights, trend analysis, and a focus on end-to-end QC processing.
These advantages ensure correct analysis and prompt corrective actions. Ultimately, they drive 100% regulatory compliance, which improves regulatory decision-making on public health issues. By enabling safe drug use and safeguarding patient health, ACQC helps us all live better lives.
References:
- Webinar Q&A: Importance of Quality Case Processing in Pharmacovigilance Operations.
Importance of Quality Case Processing in Pharmacovigilance Operations (sequrelifesciences.com)



