The unprecedented power of AI is transforming the Life Sciences (LS) industry across the pharma value chain from research and development and clinical trials to pharmacovigilance. The impact and value seen by implementing AI have driven LS companies to be proactive in acquiring and quickly adopting these tools for fear of missing out.
As AI continues to evolve, ethical considerations, data privacy and regulatory challenges must be carefully addressed to ensure responsible and trustworthy AI implementation. AI applications within the LS sector must respect the data privacy concerns raised by regulatory authorities, the industry in general and patients.
Fusing AI with LS data presents many ethical questions that require a well-documented and approved strategy before adoption.
- Is real-time data available?
- How compatible is the LS dataset with the desired or the actual solution?
- Is the data quality good enough?
- Is the data volume enough for analysis?
- Is the data safe, secure and aligned with regulation requirements?
This blog examines the characteristics of the LS dataset, LS data impediments and what it will take to advance AI/GenAI adoption in the life sciences industry.
Data across the LS value chain and its constraints
The life sciences industry is overwhelmed with data, as it has an abundance of diverse data types from multiple sources at every step in the pharma value chain.
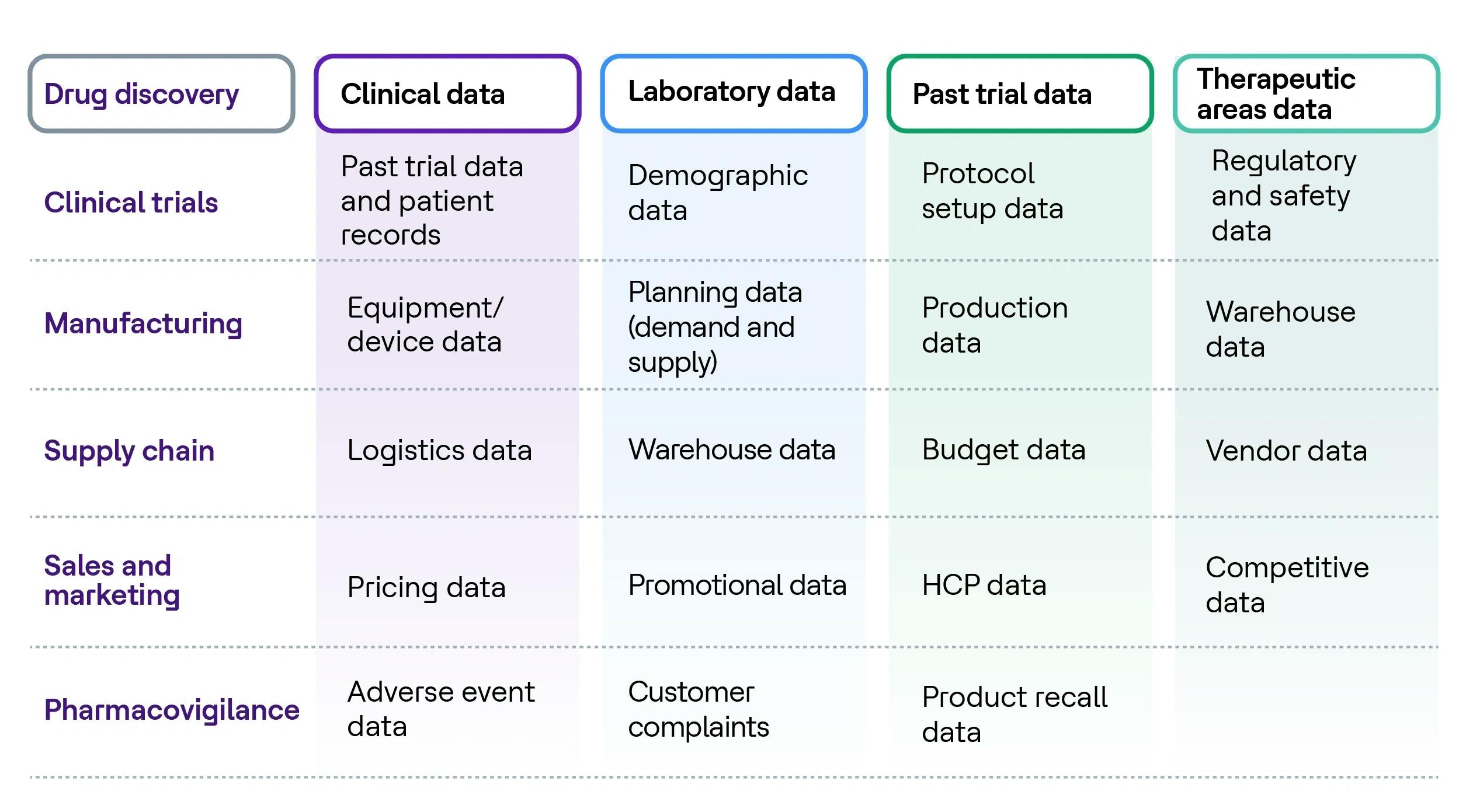
The LS industry has yet to realize the benefits of data due to a lack of high-quality data, data interoperability and inefficient data management.
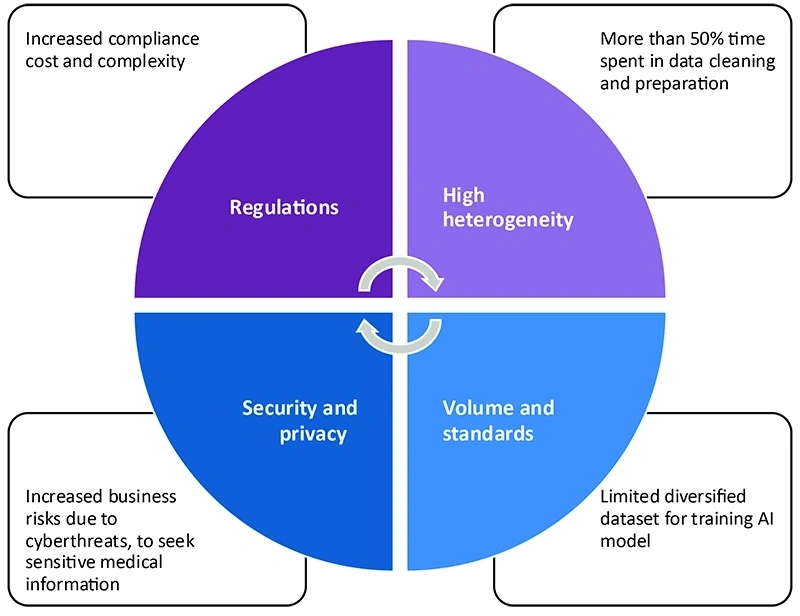
Data challenges in the life sciences industry
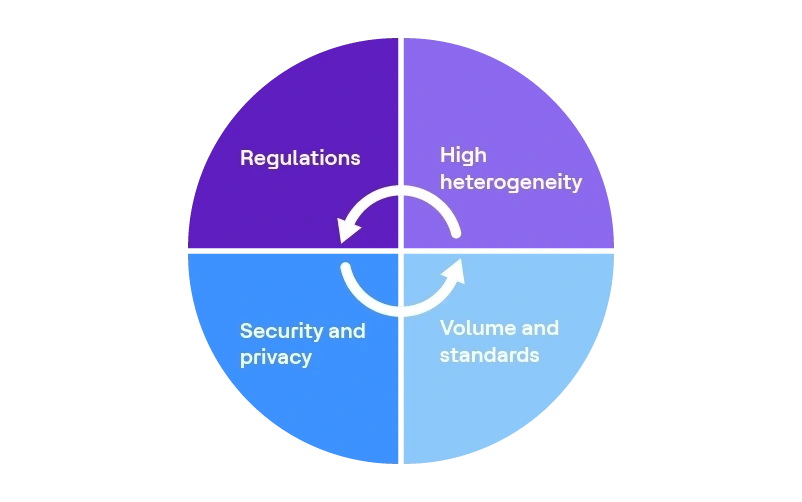
- High heterogeneity
- The data comes from multiple disconnected sources and arrives in different shapes and sizes. For example, in R&D data there is an integration of data from disconnected sources with data from patients, payers and healthcare professionals through different channels.
- Volume and standards
- The vast quantities of data come from patients, providers and payers in various formats (text/video/images). In addition, data formats are inconsistent, unstructured and without any data standards.
- Data availability and accessibility
- While all the above is true, data in LS is not easily available and accessible to anyone due to data security, patient safety and regulation requirements.
- Regulations
- LS organizations need to comply with numerous industry-specific regulations in areas like data collection, storage, transmission and sharing.
- Additionally, they need to comply with regional regulations such as European GDPR (General Data Protection Regulations), HIPAA (Health Insurance Portability and Accountability Act), CCPA (California Consumer Privacy Act) and GxP (Good Practices).
The impact of life sciences datasets on AI/GenAI
Due to the inherent nature of LS data, the life science industry is struggling to unify data silos and develop data governance models to make it compatible with AI model training.
In addition, a common challenge in AI is data bias (not specific to LS alone), i.e., the reliability of the dataset or the perceived source of truth. To ensure that biases are well-managed, any new technology must be “inclusive.” This can be done only if the AI is trained on an accurate representative of the diversified dataset.
- If the datasets contain biased information, the model will also exhibit the same bias when making decisions.
- A lack of LS data and biased data aggravates the risks exponentially, especially when the AI model is integrated with sensitive applications.
Robust data strategy for trustworthy AI development – the way forward
A clear data strategy is necessary for true AI value to become leaner, faster and more efficient.
- Dataset curation
- Utilizing the right data quality and volume dataset to train the AI against vast data that might or might not be available
- Cleaning and harmonizing the data for interoperability
- Reducing data bias
- Implementing an effective dataset design
- Ensuring data integrity (accuracy, completeness and quality over its entire lifecycle)
- Test data management
- Data testing for quality, fit for purpose and availability when needed
- Data governance
- Implementing a data governance framework
- Utilizing data security guardrails for data storage and transmission
- Properly managing data access
- Regulatory compliance
- Implementing key measures and controls, adhering to guidelines from GDPR, regulations and the upcoming EU AI ACT (for responsible AI)
- Leveraging existing defined standards from NIST
- Ensuring policies are in place to ensure ethical practices
- Implementing robust compliance programs, audits and proactive measures
- Data documentation for future AI audits
Conclusion
Leaders must take incremental steps to transition into data-driven organization to unearth insights across every value chain element such as:
- Effective collaboration between LS leaders as well as leaders from other industries
- Creating a clear AI strategy, including people, processes and technology
- Partnering with hyper-scale service providers to leverage AI for the creation of data (synthetic data) and proof of concepts (POC)
- Developing a process for the continuous monitoring of data
HCLTech’s comprehensive suite of services and solutions, including validation, regulatory, cybersecurity, data and domain-focused teams, is designed to help companies accelerate efforts to implement transformational AI/GenAI use-cases.


