Clinical trials hold the key to medical advancements, but navigating the supply chain of investigational drugs and materials can feel like walking through a dense jungle. A lack of visibility, delays and discrepancies can stall research and jeopardize patient safety. This is where SAP Integrated Clinical Supply Management (ICSM) steps in, helping to clear obstacles and bring light to this complex process.
This blog shares insights into what SAP ICSM is and why it has become game changer for Life Sciences companies in the clinical trial space. Initially released in 2022, SAP ICSM was designed and has since evolved based on feedback from a consortium of over 25 Life Sciences organizations operating in the clinical trial industry. This collaboration has resulted in an advanced product that addresses the industry's need for transparent, flexible and integrated clinical trial supply management.
The depth of industry involvement means that SAP ICSM is fast becoming the gold standard in clinical trials. For sponsors, CROs and research institutions, investing in SAP ICSM solutions represents a commitment to efficiency, safety and ultimately, the success of their clinical research endeavors.
So, what is SAP Intelligent Clinical Supply Management (ICSM)?
SAP ICSM is a cloud solution that automates and orchestrates the entire supply process related to clinical trials, from forecasting and procurement to labeling, packaging, distribution and inventory management. It is an add-on to SAP S/4HANA, using SAP Cloud Connector to connect SAP ICSM and an SAP S/4HANA on-prem instance.
Imagine a unified control center coordinating every step, ensuring the right drug reaches the right patient at the right time, all while maintaining regulatory compliance and patient safety. SAP Intelligent Clinical Supply Management allows clients to manage the planning, sourcing, manufacturing, distribution and reconciliation of supplies for clinical studies and addresses specific blinding and randomization needs.
SAP ICSM has four embedded modules covering demand and supply, including:
- Two cloud modules of SAP Intelligent Clinical Supply Management based on SAP Business Technology Platform (BTP), which cover study management, planning and demand forecasting.
- Two modules of SAP ICSM are part of an SAP S/4HANA Life Sciences add-on. This is integrated with the SAP S/4HANA instance for actual execution purposes and covers the specific blinding and randomization needs for clinical trials to facilitate manufacturing, packaging, labeling and shipments of clinical supplies.
To fully grasp SAP ICSM’s capability, it’s important to understand its integration points and the functionalities it offers across the end-to-end clinical trial supply chain process.
SAP ICSM seamlessly integrates with several applications, including SAP S/4HANA, through standard SAP API’s. This is validated through SAP ICSM’s overall architecture (shown below), which is based on a Cloud Connector add-on that integrates the SAP ICSM cloud module on SAP BTP with the SAP S/4HANA instance. The SAP ISCM module is used for clinical planning and forecasting, while the SAP S/4HANA instance is used for commercial supply chain planning.
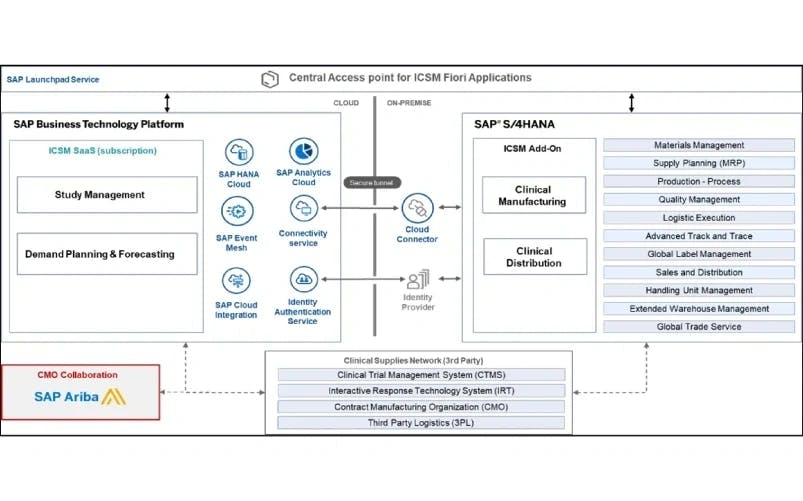
Ref: SAP ICSM release notes
SAP ICSM’s real superpower is its seamless integration with key business solutions, listed below. It is this integration that allows it to address current challenges in clinical trials management head on — and what sets it apart from other management systems on the market.
- Clinical Trial Management Systems (CTMS): These platforms act as the central hub, integrating data from various sources and providing a holistic view of the supply chain
- Warehouse Management Systems (WMS): Optimize inventory control and distribution, ensuring accurate tracking and temperature-controlled storage
- Interactive Response Technology (IRT): Facilitates randomization and blinding while maintaining patient safety and regulatory compliance
- Data Analytics: Powerful insights inform forecasting, identify potential issues and drive continuous improvement
Addressing clinical trials supply chain management’s key challenges
Understanding how SAP ICSM tackles current clinical trial challenges highlights its competitive edge. The clinical industry has long relied on using several siloed applications along with manual intervention to perform the activities across the entire supply chain (both commercial and non-commercial). This has resulted in delays to clinical trials that have a domino effect on go-to-market strategies.
To start, the fragmented nature of clinical supply chains often leads to isolated data silos and increased complexity. Each stakeholder may operate with its own system, creating a labyrinth of disconnected information.
Regulatory challenges add another layer of complexity, as the stringent requirements for handling and distributing investigational drugs vary across regions and change frequently. Patient concerns are paramount, as any lapse in the supply chain can directly affect their safety and the trial’s integrity.
The trial period itself is often a race against time, with delays potentially stalling life-saving treatments. And finally, budgets must be meticulously managed, as inefficiencies and wastage can lead to exorbitant costs.
SAP ICSM, however, addresses these common challenges and more, which are sure to look familiar to those in the industry:
- Integration of multiple siloed applications
- End-to-end visibility of the supply chain (both commercial and clinical)
- Managing and enhancing the patient experience
- Optimizing trial period duration
- Efficient utilization of clinical trial budgets
- Timely availability of dosage for clinical trial patients
- Reducing time and effort for data reconciliation and reporting
- Timely, accurate reporting to meet regulatory needs
Harnessing the power of integration
This is where SAP ICSM becomes a game changer. By integrating all aspects of the clinical supply chain into a cohesive system, SAP ICSM addresses these challenges head-on. It breaks down data silos, providing a single source of truth that enhances visibility and traceability across the entire supply network. Real-time data integration allows for better forecasting and alignment of supply and demand, reducing the risk of shortages or overstock.
Moreover, SAP ICSM streamlines regulatory compliance by automating documentation and ensuring adherence to the latest guidelines. This not only mitigates the risk of costly compliance breaches but also accelerates the approval processes, keeping trials on track.
Patient safety, at the core of clinical trials, is bolstered by SAP ICSM through its rigorous quality control measures. The system ensures that every step of the supply chain — from procurement to delivery — is meticulously monitored and documented, maintaining the integrity of the investigational drugs.
Financial efficiency is another critical benefit. By optimizing inventory levels and reducing wastage, SAP ICSM drives down costs, making clinical trials more financially viable. This, in turn, allows for the allocation of resources to other vital areas of research and development.
In essence, SAP ICSM transforms the clinical supply chain into a well-orchestrated symphony, where every component works in harmony to ensure the success of clinical trials, resulting in more efficient, cost-effective and patient-centric research — and ultimately accelerating the delivery of new treatments to those in need.
Key features and benefits of SAP ICSM include:
- Centralized supply chain management: SAP ICSM provides a single platform to manage all aspects of the clinical supply chain, from inventory, logistics and patient supply.
- Real-time enhanced visibility: Real-time insights into inventory levels, location and temperature conditions offer peace of mind and allow for proactive decisions. This helps in identifying potential issues before they can impact the clinical trials.
- Streamlined efficiency: Automated processes and integrated systems reduce manual work, minimizing errors and saving time.
- Cost reduction: Optimized inventory and minimized wastage lead to significant cost savings, benefiting both sponsors and research institutions.
- Improved patient safety: Strict oversight and adherence to regulations ensure that the drugs patients receive are of the highest quality and potency. Timely delivery of investigational products and minimizing supply shortages ensure improved patient safety.
- Faster trial times: Reduced delays in the supply chain, leading to faster trial completions and accelerated time to market.
- Regulatory compliance: Enables adherence to global regulatory standards and maintaining accurate documentation with minimal manual intervention.
- Reconciliation and reporting: SAP ICSM provides the ability to generate comprehensive reports on supply chain performance, cost and compliance. It also helps in reconciling inventory and patient supply data.
HCLTech: A partner of choice for your SAP ICSM journey
SAP ICSM empowers pharmaceutical companies to streamline their actual clinical supply chain operations and accelerate drug movement. Drawing on HCLTech’s comprehensive Life Sciences industry expertise, including a Life Sciences Clinical Trial CoE, SAP BTP expertise, innovative solutions, frameworks and insights, we can help completely transform your current supply process for clinical trials with SAP ICSM. To help accelerate and derisk this journey, we offer a comprehensive jump start offering designed for rapid and seamless SAP ICSM implementation. This includes process accelerators with clinical trial business processes mapped in SAP Signavio (used for Process mining) and pre-configured SAP demo instance to help accelerate the discover phase of the program. To learn more about our SAP ICSM jump start offering, consulting services or to speak with a member of our Life Sciences team, please contact us at SAP@HCLTech.com.


