Introduction
In the pharma manufacturing domain, whether it's primarily bulk drugs – active pharmaceutical ingredients (APIs), biomanufacturing, formulations, or medical devices, the role of sensors or endpoint computing machines and devices is very crucial from a quality, safety, and yield perspective. With the broader adoption of Industry 4.0 frameworks and innovations in the Industrial Internet of Things (IIoT), these devices have started taking center stage in the IT transformation roadmap. The manufacturing devices which bring computing right to the point where data is generated at the shop floor or plant are called Edge devices. There has been a lot of buzz around "cloud computing" but "Edge computing" is equally gaining mindshare in the pharma manufacturing space.
A massive amount of data is generated on the shop floor based on real-time operations and compliance requirements. Sending all that data to the cloud may be helpful from enterprise data analytics standpoint but adds to the latency in sending information back to the shop floor. Edge devices are now powerful and capable enough to analyze data on the shopfloor and derive meaningful insights into manufacturing operations. These IIoT devices provide production data and provide diagnostic information about their health.
Industry trends
Gartner's IoT forecast shows that by 2029, more than 15 Billion IoT devices will attach to the enterprise infrastructure. IDC predicts that the number of apps at the Edge will increase 800% by 2024. These Billions of Edge devices have the potential to provide revolutionary changes in the pharma manufacturing space. Coupled with innovation in the 5G network, it is bound to create new use cases which could not have been envisaged earlier.
Edge architecture strategy
In the life sciences industry, the collection and retention of data from the Edge architecture are paramount and are used to meet regulatory compliance. The architecture must be agile and distributed to meet diverse production and manufacturing scenarios. A typical Edge architecture would have multiple smart IIoT sensors. Edge devices talk to each other, analyze data packets at the source, and derive critical operational insights.
High-level architecture
Edge computing architecture
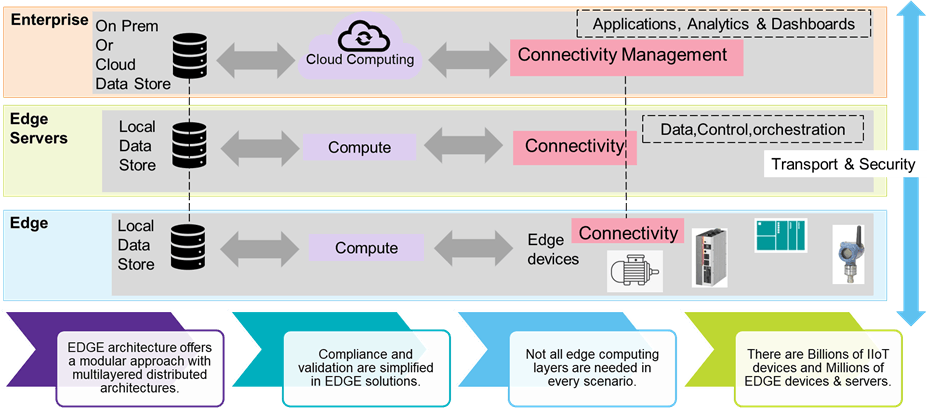
Instead of sending shop floor data to the cloud, the architecture has servers on the shop floor collecting and analyzing data very near to the point of generation, called Edge servers. Since these servers are not hosted in the cloud, they offer "low latency" benefits and higher processing power for larger data chunks than IIoT field devices. Technology plays a crucial role in defining the Edge architecture. Some can be web-based applications loaded onto the Edge server(s). Others can be very lightweight applications designed for deploying at IIoT or edge devices. Very light applications for digital validation need to be considered in the Edge strategy. The application of 5G wireless networks is still nascent, but we can foresee significant development in this space. Understanding the user requirement specification holds the key to defining the Edge architecture.
Challenges in Edge deployment
Not everything is a cakewalk for Edge computing in pharma manufacturing. There are several challenges around how the data would be retained in a regulated environment. These Edge devices should follow the 21 CFR Part 11 guidelines, considering two factors:
- the small storage capacity they inherit as part of compact standalone solutions
- the complex issue of addressing the real-time data accumulation at Edge locations and devices
Some manufacturing locations are remote, and Edge devices can even perform in isolation without connectivity to the internet, cloud, or data center. But, in case of hardware or software failures, there are operational challenges to be addressed, such as servicing these devices at isolated remote locations. Edge works on a distributed architecture, and each of the Edge devices or IIoT devices must talk to each other for an integrated solution at the Edge location. Having a standardized, unified architecture with adequate bandwidth for reliable communication will lead to a successful Edge strategy.
Key Edge players in the industry
All big technology organizations are investing heavily in Edge computing, such as IBM, Intel, VMware, Siemens, Rockwell Automation, etc. While new technologies are under constant development, a common standard and a consortium that governs these technologies, shall will be beneficial be formed for a holistic adoption of Edge in the manufacturing or production shop floor.
Benefits of Edge in pharma manufacturing
All set aside, the benefits of Edge computing in the pharma manufacturing space can outstrip the challenges faced by the technology.
- Faster response times: The biggest strength of Edge technology on the manufacturing shop floor is its sharper response times. The loop cycle time for information processing, analytics, and visualization is way faster than what cloud technologies can offer. It makes for faster operations and compliance on the manufacturing shop floor and reduces Batch cycle times.
- Security: The exposure of Edge data is mostly limited within a confined plant or operating perimeter, and hence they are less susceptible to cyberattacks. Many Edge and IIoT devices are working on industrial-grade communication protocols with several layers of protection for inter-device connectivity in a distributed architecture.
- Compliance: Most smart Edge devices and IIoT products are evolving very fast, and in the pharma value chain, their acceptability lies in their ability to provide audit trail, data integrity, and data portability capabilities. These devices are compatible with North America (NA) and European Union (EU) regulatory requirements and can port the data out for long-term archive as per regulatory data requirements.
- Scalability: Edge offers a highly distributed architecture with the ability to scale from small unit operations to enterprise-wide deployments. Containerized applications offer personalization to the Edge devices and help improve operational efficiency. Edge devices can perform on their own even if they are not connected to the internet for performing the assigned tasks. Artificial intelligence & machine learning (AI/ML) also improve the asset performance of the various machines or equipments.
- More vivid AR/VR: With faster processing on the ground and robust visualization tools based on augmented reality (AR) & virtual reality (VR) it can offer significant opportunities for pharma manufacturers to change gears. Instead of simple logbooks for knowledge transfers (KT), AI/ML provides tools for both seamless KTs and improving overall equipment effectiveness (OEE). AR/VR-based remote maintenance and operational workflows have significantly reduced downtimes and increased manufacturing efficiency.
- Lower costs: The Edge computing technology is relatively lean compared to its cloud computing cousin. There are no central servers and large networks sending data from the shop floor to data centers across the globe. Securing these shopfloor Edge networks are much simpler. The total cost of ownership (TCO) is significantly lower. It provides lower maintenance costs, alerts, and decision-making in real-time.
Conclusion
Edge computing can let manufacturers take significant advantage of Industry 4.0 in pharma. However, a comprehensive strategy with a mix of Edge and cloud should span across enterprise architecture. The core values Edge technology offers are significant improvements in reducing batch cycle times, digital compliance, and paperless operations. The transition to Edge has already started across the industry and will catch a more incredible pace in the coming years. New Edge/IIoT devices would open many more use cases across Life sciences manufacturing. Though its adaptability depends on how relevant those use cases are to the industry needs and how well ROI expectations are met for the pharma manufacturers.
References
- Jeffrey Palmer (2020, February 19). Why Edge computing needs Autonomous Management. IBM Website. Retrieved February 27, 2022, from https://www.ibm.com/cloud/blog/why-edge-computing-needs-autonomous-management
- David H.Deans (2021, April 9). How cloud edge and IoT will improve pharma manufacturing. CloudTech. Retrieved February 26, 2022, from https://cloudcomputing-news.net/news/2021/apr/09/how-cloud-edge-and-iot-will- improve-pharma-manufacturing/